Description
MXene Ti3C2 Materials
We provide world class MXene materials. Used by top researchers and scientists around the world. Try some today for applications requiring high conductivity! Excellent results in conducting additives widely used in batteries, supercapacitors, and electrodes. MXenes are conductive, hydrophilic 2D materials so named to emphasize their graphene-like morphology. MXenes are used as electrode materials for lithium-ion batteries and supercapacitors and are promising hydrogen storage medium, lead adsorption medium, and catalysts. Sold by the gram.
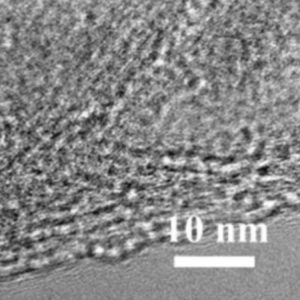
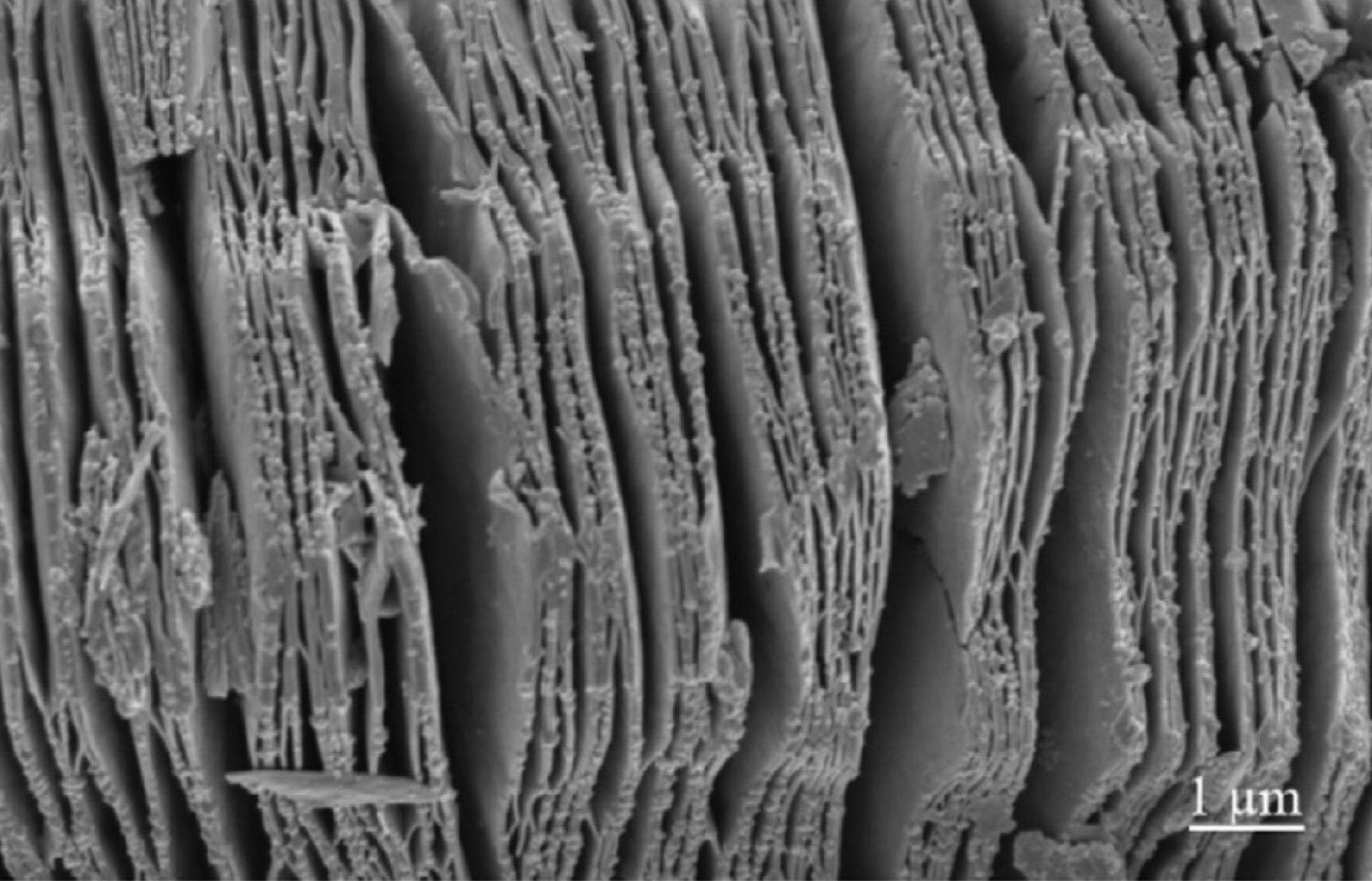
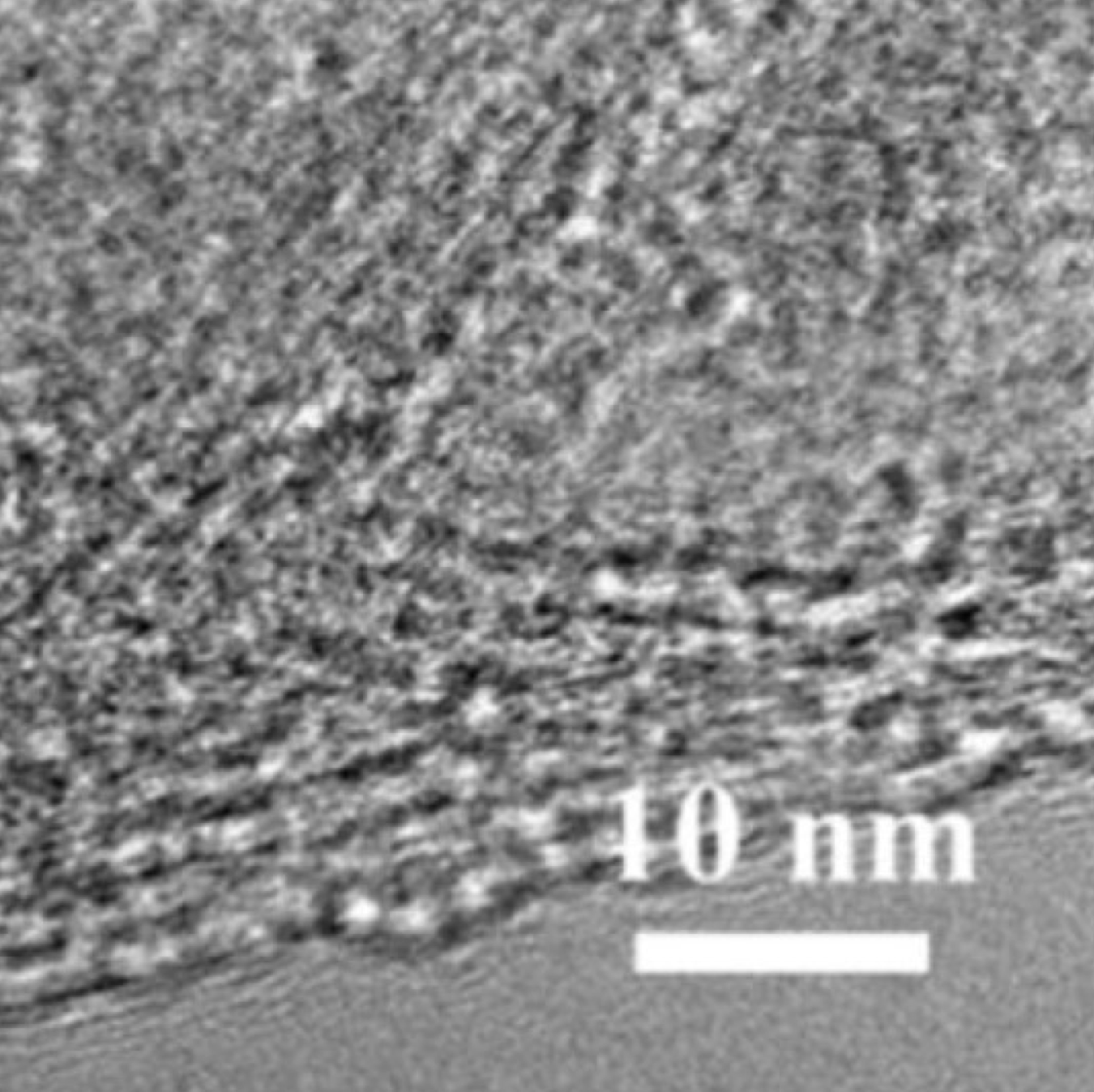
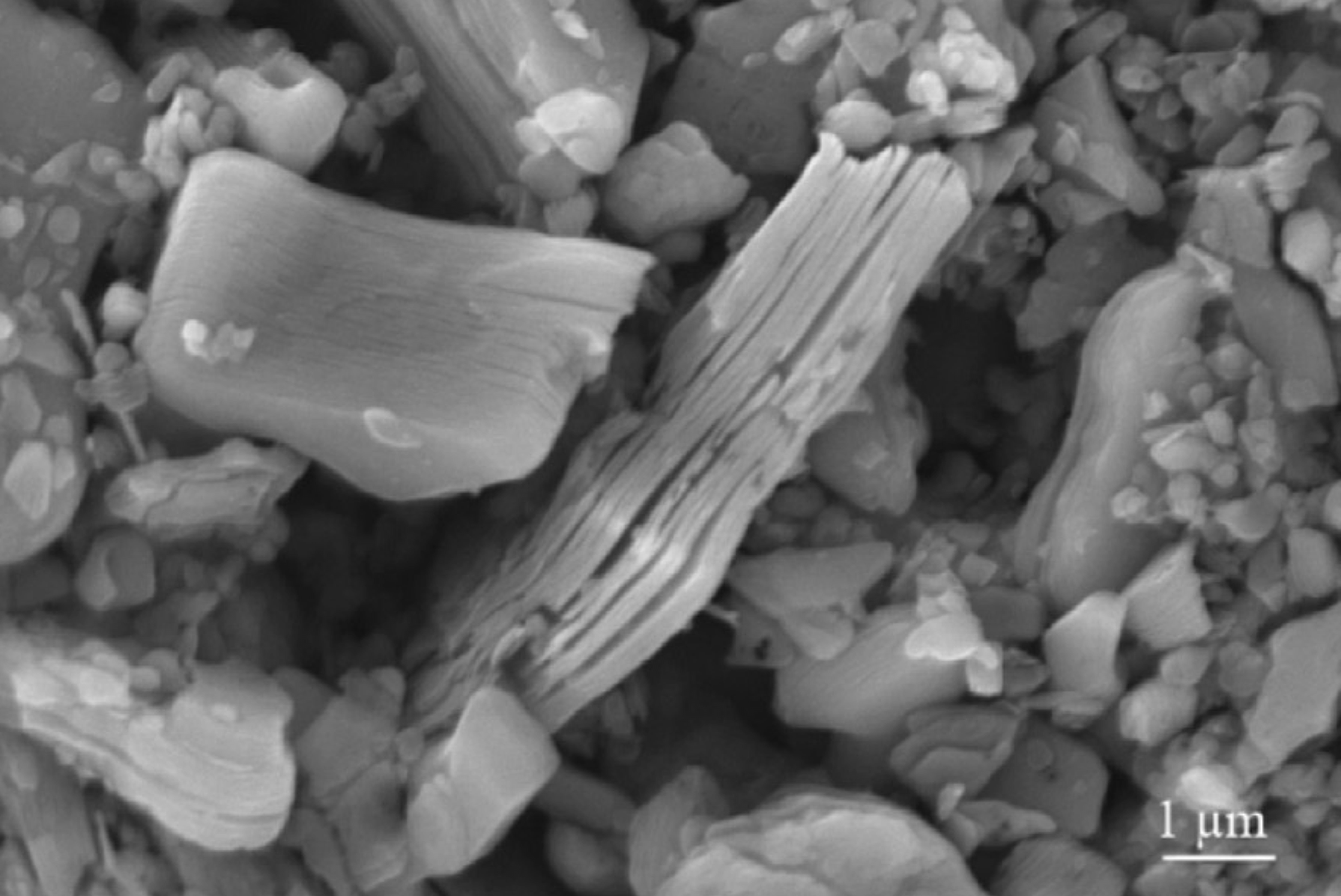
The precursors of MXenes, are carbides or nitrides with the general formula of Mn+1AXn, where M is an early transition metal; A is an A-group element (mostly group IIIA or IVA); X is either carbon or nitrogen and the value of n can be 1, 2, or 3. A typical MAX compound consists of metallic A layers and ceramic Mn+1 Xn layers. A layers are chemically more active than Mn+1Xn layers. 2D MXenes were made by removing A layers from MAX phases by HF etching. The surfaces of MXene made in HF solution are usually terminated by F and/or OH groups due to its high surface energy. shows the chemical structures of a typical MAX phase (Ti3 AlC2 ) and MXene (Ti3 C2 ) with OH termination.
| Item | Main Ingredient | Purity | State | Conductivity | Stability | BET | Dispersion |
| MXene101 | Ti3C2 + Additive
|
>99% | Black Powder | ~1200 S/cm | RT for three months | ~30m2/g | dispersed well in water or polar organic solvents |
| MXene110 | Ti3C2 + Additive + solvent (water or others) | Conc of Ti3C2 is 2% (W/W) | Black suspension | NA | RT for three months | NA | di water |

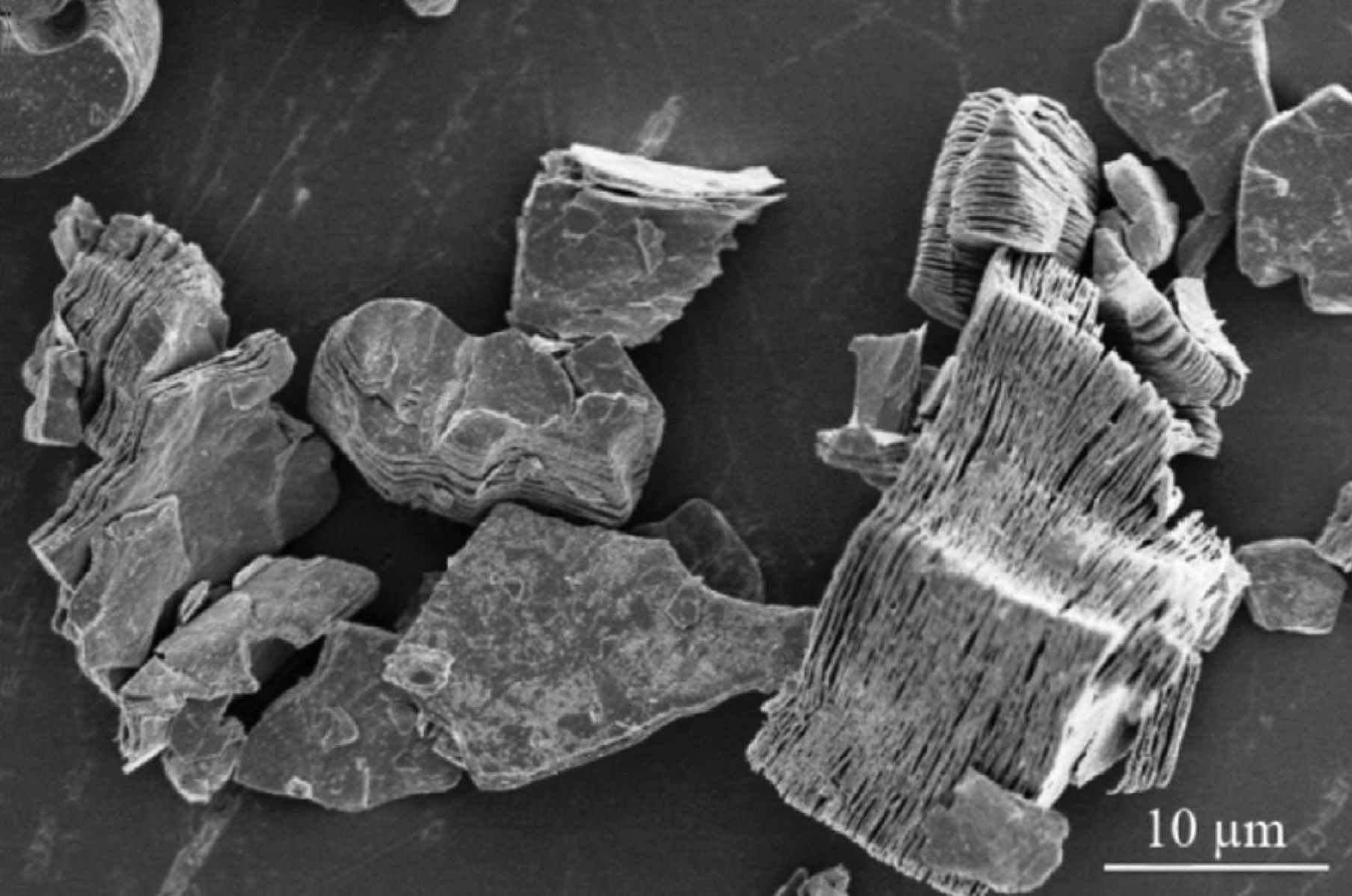
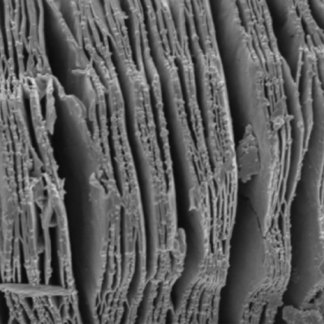
MXene Ti3C2 SEM IMAGES

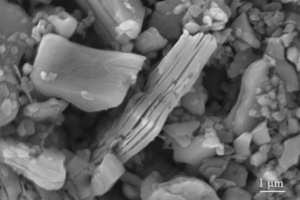
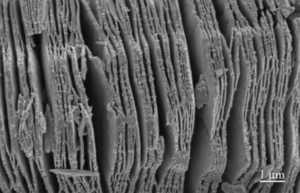
MXene Ti3C2 TEM IMAGE
MXene Ti3C2 TEM IMAGE