We are always fascinated to learn more about the production processes used fabricate nanoscale products. We grow silver nanowires and have spent significant amounts of time and money developing our process internally. This study reveals previously unknown yet essential ingredients for nanowire growth of germanium nanowires via the vapor–liquid–solid mechanism.
Surface adsorbates are well-established coordinators in material synthesis whose presence has a direct impact on semiconductor nanowire growth. Nanoscale engineering is challenging to say the least and reaction kinetics are critical. Most processes are designed to run right on the line between success and failure. The chemistry – molar ratios, raw materials as well as temperature and pressure are intensely scrutinized and rightfully so but there is an art to it as well. The art is the stuff that you typically don’t find in journal articles or what some consider to be voodoo. This study helps to pierce the veil and will facilitate a greater understanding of reaction kinetics thus giving many researchers greater control of nanowire synthesis thus helping nanoscience push the envelope which hopefully enables further commercial opportunities for germanium nanowires by facilitating a more consistent product with tighter length tolerances. Well done Saujan V. Siveram, Michael Filler, Naechul Shin, Li-Wei Chou et al.
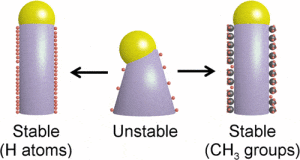
“A study out of GA Tech’s Filler laboratory has gained unprecedented insight into the nanowire growth process through the use of real-time infrared spectroscopy. The study found that surface species, specifically hydrogen atoms and methyl groups, decorate the nanowire’s surface and are essential for the stable growth of nanowires made from germanium.
Without the presence of hydrogen and methyl adsorbing (or adhering) to the nanowire sidewalls, the liquid droplet that sits atop the nanowire could slip, causing growth to cease. “These surface species, hydrogen and methyl molecules, act like a layer of Rain-X, keeping the droplet in place,” Filler explains.
“Our work shows that without these surface adsorbates, growth doesn’t happen. No one knew that before,” says Filler, whose research team published its findings in a recent issue of the Journal of the American Chemical Society. “For as long as scientists have been using this growth method – more than five decades – we didn’t know that anything was present on the wire surface.”
“The fundamental chemical knowledge provided in our study promises to advance the rational synthetic design of nanowire structure and function,” Filler says.
Titled “Direct Observation of Transient Surface Species during Ge Nanowire Growth and Their Influence on Growth Stability,” the study was led by Saujan V. Siveram (PhD 2015) who collaborated with Filler, Naechul Shin (PhD 2013), and Li-Wei Chou, a former postdoctoral researcher at Georgia Tech.”
